Evaluate Site-Specific Health Effects Data
This section describes the process to evaluate site-specific health effects data, including health outcome data, biological monitoring data, and medical data and information.
Meaningful health outcome data (HOD) or human exposure data can sometimes provide evidence—ranging from weak to strong—of plausible associations between site-specific exposures and human health effects. In some cases, site-specific biological data (e.g., contaminant concentrations in blood or urine) might offer insights on the extent of actual exposure (beyond the exposure-dose estimates generated from sampling concentrations). The toxicokinetics of the substance of interest should be well understood before making decisions based on biological data. For example, site-specific blood levels of dioxins and polychlorinated biphenyls (PCBs) can reflect long-term exposures and therefore, provide information about historical exposures, including exposures that have been mitigated. Other chemicals, such as volatile organic compounds can reach peak blood concentrations quickly and leave the body quickly so that blood or urine levels will reflect very recent or ongoing exposures. In the case of some bone-seeking metals such as lead, blood levels will reflect a short (120-day) window of exposure while bone lead levels indicate long-term exposures.
Remember: The overarching reason to evaluate HOD, if available and appropriate, is to understand if the exposed population has an elevated rate of disease as measured in existing databases.
In rare cases, individual medical reports might be available, documenting symptoms or clinical examination results. Credible reports of symptoms, illness, or disease, along with other HOD, can be used to support recommendations for public health actions, such as a health study or a biomonitoring investigation. However, there is typically a lack of local, county, or state HOD available to correlate site-specific exposure with known health effects from a contaminant and an inability to determine an exposure-response relationship. Even when data are available at the local or county level, caution is warranted in conducting an HOD review because it is not usually possible to link health outcomes with site-specific exposures. For example, HOD might be available at the census tract or county level but exposure is limited to only a small geographic area within the census tract or county. In this situation, HOD results probably should not be used to decide whether or not the exposed population is experiencing harmful effects from site-related exposure.
Health Outcome Data (HOD)
HOD are reportable statistics or information on births, deaths, diseases, and symptoms that characterize a population’s health status. ATSDR is required by the Superfund law to consider the evaluation of HOD during the PHA process. The law indicates that ATSDR should include relevant HOD analyses when exposure to site contaminants could have resulted in the development or exacerbation of health effects.
HOD reviews are population-level screening evaluations to determine if there is evidence of elevated disease occurrence within an exposed population. These reviews are usually ecologic in nature because the results cannot be used to make inferences about site-specific exposure causing the health outcomes being monitored.
If included in your evaluation, you can use HOD to do the following:
- Compare the disease occurrence between a population potentially exposed to site contaminants with an appropriate reference population (e.g., county, state, or national data). This comparison can provide insights as to whether the potentially exposed population is experiencing health effects at the same, lower, or higher rate than expected. It can also help you determine whether there are increased illness patterns in the exposed population.
- Address community health concerns about the occurrence of disease in their community.
- Identify the need for follow-up health actions such as exposure (biological monitoring) investigations, analytical epidemiologic studies, or health surveillance.
At the outset of the PHA process, the health assessor in concert with the site team should plan community engagement activities to learn about community concerns and provide information about ATSDR products and services, including the utility of analyzing HOD. Specifically, you should explain how ATSDR uses HOD, when it is available, and the criteria and rationale used to determine whether a HOD evaluation would enhance decision-making in the PHA process.
In particular, make the community aware of the strengths and limitations of descriptive epidemiologic analyses (as outlined below). Elevated disease rates alone cannot be considered conclusive evidence that living near a source of environmental contamination is the sole cause for the occurrence of the elevated rates. Health outcome or descriptive epidemiologic analyses are only an initial step in determining the nature and extent of disease in the community around a site.
Types of Health Outcome Data
- Morbidity data (such as cancer incidence, birth defects, or other diseases from state or county disease registries).
- Vital statistics data (birth and death certificates).
- Disease information from community health records, healthcare provider agencies, and individuals (such as from private doctors).
- Health statistics from community health studies.
- Other types of data such as hospital discharge and emergency department data.
The availability of site-specific HOD that are sufficient to draw inferences about their association with site-related exposures are often limited. However, when adequate HOD are available to review and analyze, they can add value to the health evaluation in the following ways:
- Focusing on a specific community with defined geographic boundaries and over a specific time.
- Helping to answer the question of whether there is more disease in a community than expected.
- Evaluating the occurrence of a specific disease outcome that may be a community concern.
- Helping determine whether a specific health effect is elevated in a particular area.
- Providing the potential for aligning exposure levels with disease occurrence.
But while there are strengths, there are also many limitations of using HOD reviews to correlate with estimated or measured exposure levels in a community:
- Lack of ability to determine a dose-response relationship between exposure and health outcome
- Long latency period for some outcomes (e.g., cancer)
- Confounding risk factors that might be the real cause for an increased rate
- Lack of data for many diseases
- Low statistical power of correlating exposure with diseases that have low incidence rates
- Inability to establish a specific cause for an observed effect
Important: Health outcome data are rarely available or of sufficient quality to associate health outcomes with site-related exposures. Health assessors need to use ATSDR’s specific criteria to determine whether an HOD evaluation is possible, and if so, decide if it is appropriate.
Deciding Whether and How to Use Health Outcome Data
Work with your team to decide how to use or analyze HOD—or whether to use it at all. Work with epidemiologists, and gain input and feedback from statisticians, toxicologists, community involvement specialists, health educators, and environmental scientists. The following six questions can help guide your team in making the decision (also see the decision tree diagram). These questions focus on site-related exposure considerations only. You need to answer “yes” to all six questions before you can consider analyzing HOD and discussing it in your public health document.
If you answer “yes” to all the questions, the team should seek help from an epidemiologist or statistician knowledgeable in analyzing HOD to determine if this type of evaluation is feasible based on various factors (e.g., size of exposed population, availability of data). A HOD analysis may be of assistance in the overall assessment of potential health impacts to the site community. However, given the uncertainties and limitations, it is not considered to be a requirement for inclusion in all ATSDR public health documents. Depending upon the complexity of the analysis, you may decide that an HOD analysis warrants a stand-alone public health document (e.g., a PHA focused solely on the HOD analysis).
Six Questions to Ask When Deciding Whether to Conduct a Site-specific HOD Analysis
- Are there one or more current (or past) potential or completed exposure pathways at the site? A HOD evaluation will only be helpful in assessing potential harm related to the site if there is at least one potential or completed exposure pathway.
- Can you determine the extent of exposure (e.g., duration, concentration, location)? A site-related HOD review will only be helpful if there is at least an estimate of the exposure characteristics, including where (residential vs. occupational), how much (concentration), how long (duration), and how often (frequency) exposure has occurred.
- Note: You need a reasonable estimate of the duration of exposure to determine whether the HOD being analyzed could be the result of exposure to site contaminants. The relevant exposures could range from a few days to many years before the onset of disease, depending on the nature of the adverse response, mode of action of the contaminant involved, the dose or concentration, age of the individual exposed, specific health outcome, and other factors. Make sure that the available HOD are from the time when site-related health effects are likely.
- Can you quantify the size of the exposed population, either past or current? You will need a reliable estimate of the number of people exposed and the total number of people in the study population. The availability of demographic information within the exposed and non-exposed study population (e.g., age, number of years at residence, smoking status) is also an important consideration. If you cannot make this estimate, you do not need to analyze site-related HOD.
-
- Note: To calculate the rate of health outcomes among the exposed population, you need data on (1) the number of people who have certain health outcomes in a selected population, and (2) the estimated number of people exposed. This information is required to adjust the incidence or prevalence of mortality or morbidity rates in the exposed population to a non-exposed comparison population. Based on this, you can determine any differences in disease rates between the exposed and non-exposed population.
To identify the exposed population, you need information on where (i.e., the geographic extent) exposure occurred. Analyzing HOD could be impractical in sparsely populated areas because the population is too small to measure a reliable disease rate. For example, if the “expected” rate for a particular disease is 5 in 1,000,000 and the exposed population numbers only 100, the absence of this disease over a short time in the exposed population does not provide much perspective. Moreover, if the disease of interest is rare, you could require a large population or, at the very least, several years of morbidity or mortality data for any useful interpretation. Alternatively, one or two cases of a rare disease in a small exposed population does not automatically link the exposure to the disease. You will need to know the time period in which the cases occurred and any known risk factors present in the exposed population.
- Are the estimated exposure doses(s) and the duration of exposure sufficient for a plausible, reasonable expectation of observing an associated health effect? Analyzing site-related HOD is not scientifically reasonable unless you can make a quantitative, or at least a qualitative, estimate of exposure doses or concentrations. If you cannot make even a qualitative estimate, you do not need to analyze site-related HOD.
- Note: Qualitative exposure estimates come from exposure scenarios in which strong circumstantial evidence suggests that exposure occurred for long enough and at a sufficient dose or concentration for health effects to be possible. Such evidence could include monitoring data from nearby areas, violations of air-release or water-discharge permits, reports from residents, observations by the health assessor or other knowledgeable individuals, or other relevant information. Qualitative estimates should be based on more than one type of evidence and should be made in consultation with knowledgeable environmental staff.
An HOD analysis is most likely to be informative if the estimated past or current exposure doses or concentrations are above known effect levels for the contaminants being evaluated. HOD reviews (e.g., cancer incidence analyses) are not intended to determine an exposure-response relationship.
- Are HOD available at a geographic scale (i.e., county, zip code, census tract, or census block) to allow for inclusion of the exposed population? You need to be able to make an approximation of the size of the exposed population, based on the data source being utilized. If the data are too limited to make this estimate, you may not need to analyze site-related HOD or conduct further analysis.
- Note: To assess potential site-related effects, you need to be able to separate the health outcomes for the exposed population from the unexposed population (at least as much as possible). If the HOD available to calculate the disease rate is for an area much larger than the area exposed, then exposure misclassification bias will be introduced—resulting in severely underestimated disease risks. For populations with past exposures, a site with high population turnover (in- and out-migration) could be the basis for not analyzing HOD because of the possibility of misclassification.
- Is there epidemiologic or toxicologic evidence that exposure to the site contaminants is associated with the specific health outcomes for which there are data for the exposed population? When analyzing HOD that could be site-related, focus on specific, sufficiently known (or suspected) health outcomes in the available morbidity or mortality databases (e.g., specific cancers, specific birth defects).
- Note: The health outcomes likely to occur from exposure to site contaminants might not be in the available databases. For example, if exposure to a contaminant is linked to birth defects but not to cancer, it is not appropriate to evaluate cancer data because they are available and birth defects data are not.
In some cases, community concern about illness in the community is a sufficient trigger to pursue HOD. Assuming data are available for the disease(s) of concern and the geographic unit under evaluation, a HOD evaluation could help determine whether disease rates are elevated in a geographic area covered by the HOD in available databases. Even without a site-specific link, information about the presence or absence of elevated disease rates could either help allay fears or identify a disease trend in a geographic area warranting follow-up.
Regardless of the path you follow, your document should include a brief description of how HOD were considered, and clearly describe the reasons why a HOD analysis was or was not included. If an analysis was conducted, include a concise description of the methods used and the results and limitations.
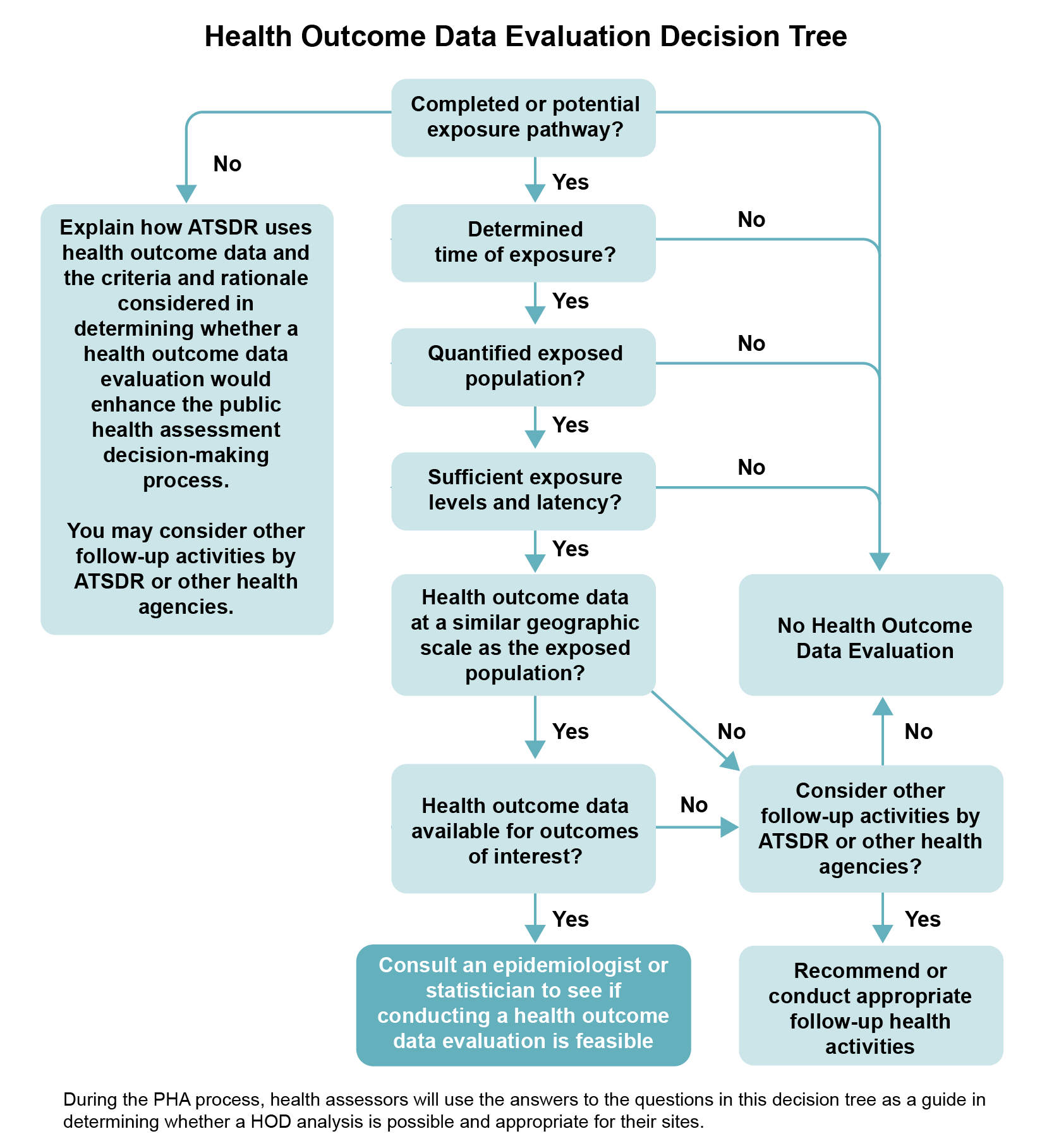
Description of Health Outcome Data Evaluation Decision Tree
Biological Monitoring Data
In some cases, biological monitoring data might be available to further define or quantify exposures to site contaminants and provide additional evidence when evaluating potential health effects. ATSDR will sometimes perform an exposure investigation (EI) to collect biological monitoring data to fill an identified critical data gap. See the PHAT EI Mini-Module [PDF – 1,034 KB] for more information on ATSDR’s EIs.
Depending on the biomonitoring levels detected, it may support or disprove plausible biological outcomes given a sufficiently large data set. Interpret site-specific biological sampling results with caution and consider the following:
- Biological monitoring data, like environmental data, need to be collected using standard procedures and analyzed by methods that are compatible with those used for collecting comparative data (e.g., CDC’s National Health and Nutrition Examination Survey [NHANES] data, clinical reference levels).
- Detected levels might not be the result of site-related exposures (e.g., increased blood lead levels could be the result of lead paint exposures or traditional medicines rather than from soil contamination).
- For chemicals with short biological half-lives, results will likely only represent recent exposures.
- For chemicals with long biological half-lives, results will not provide specific information about when exposure occurred.
- The correlation between detected levels in bodily fluids (e.g., blood and urine) and clinical effects might not be understood.
- The people tested might not be representative of the exposed population (i.e., results from a small sample group may not reflect the range in exposures across the entire exposed population).
Biological testing is most commonly conducted using blood or urine samples. When biomonitoring data are available, they can provide additional perspective for the health assessor. For some contaminants (e.g., lead), you are able to compare measured biomonitoring levels to biomonitoring levels shown in the literature that are associated with overt clinical effects from case studies or more subtle effects that might be inferred from population-based studies. Useful information sources on biomonitoring data include ATSDR’s Toxicological Profiles (sections related to biomarkers) and Case Studies in Environmental Medicine. In addition, human exposure data for selected environmental contaminants are being collected by the CDC’s National Biomonitoring Program (NBP) as part of the CDC’s National Health and Nutrition Examination Survey (NHANES). Consult with medical professionals and toxicologists for guidance in interpreting the health significance of biomonitoring data.
ATSDR’s Case Studies in Environmental Medicine are a series of self-instructional modules designed to increase the primary care provider’s knowledge of hazardous substances in the environment.
CDC’s NHANES reports biomonitoring data for more than 350 environmental chemicals in the non-institutionalized, civilian U.S. population. These data are valuable in comparing an individual’s exposure to a chemical to exposure levels in the general U.S. population.
Medical Data and Information
ATSDR does not solicit personal medical data as part of the PHA process, but sometimes citizens provide this type of information relating to their health concerns. Thus, during the PHA process, ATSDR may receive medical data, such as individual medical reports or logs of health conditions reported by community members. These data can provide some additional insights to health issues in the site community but must be used and interpreted with caution. First, if the data are privileged or confidential, take precautions to respect the individual’s right of confidentiality and privacy (see text box below). Second, the documentation of a particular medical condition in an individual(s) does not inform you of causes or patterns of disease in the community. Identify plausible biological links between exposure and reported medical concerns.
Confidentiality and Privacy Issues
Some of the data ATSDR collects during the PHA process, such as medical data, might be considered confidential or sensitive and may contain personally identifiable information (PII). Medical facilities and state health departments generally have strict requirements for handling confidential medical information. Health assessors are not typically required to handle this type of information as part of the PHA process and would more likely be reviewing environmental and exposure data and aggregate HOD (e.g., from cancer registries). But when health assessors do need to present sensitive information necessary for answering public health questions, they must do so in a way that protects the confidentiality and identity of the people involved. Health assessors should collect the minimum amount of PII and sensitive data that are necessary for the PHA process and apply enough security safeguards to protect the confidential information.
Those with access to confidential information must comply with all applicable federal laws and regulations related to privacy. Health assessors should consult with ATSDR program leaders, privacy experts, and/or legal counsel before handling any data in which confidentiality may be an issue. Health assessors can use and share the PII and confidential information only for approved purposes, and must ensure they do not disclose any sensitive information in written products and other communications (e.g., meetings, telephone calls) that could identify individual data subjects without consent.