Review Other Contaminant-Specific Toxicological Information
This section provides an overview of how to examine factors that influence whether an exposure to a contaminant could produce harmful health effects and how these factors weigh into your public health conclusions.
Source: ATSDR 2002
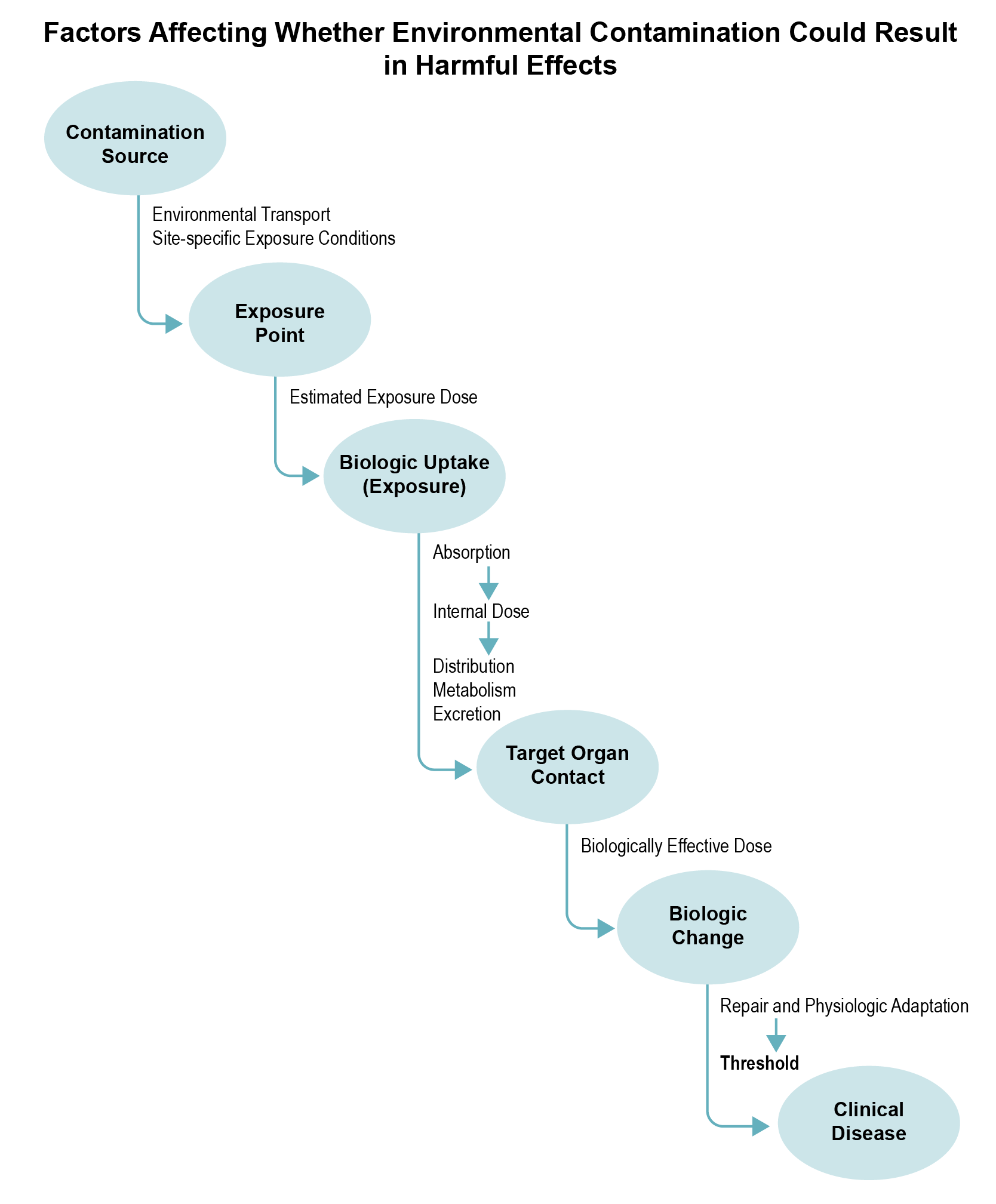
A contaminant will produce adverse or toxic effects only if it (or its metabolites) reaches specific sites in the body at a dose and over a duration sufficient to produce an adverse non-cancer effect. Whether an exposure could lead to an adverse health outcome depends on the characteristics of exposure and the exposed population (e.g., developmental stage, existing disease state, genetic factors) that could make them more susceptible to site-related exposures. The information you will need during this part of the PHA process will vary by the contaminants of concern, site-specific exposures, and particular concerns related to the site.
Toxicokinetics
The toxic effect of a contaminant will be influenced by its toxicokinetic or pharmacokinetic properties (e.g., rates of absorption, distribution, metabolism, and elimination). If available, you can obtain such information from the Toxicological Profile or other data sources (e.g., EPA IRIS). If the information is pertinent, determine what is known and unknown about the extent to which a contaminant is absorbed, as well as how it is distributed through the bloodstream, changed to different forms, excreted, or ultimately delivered to target organs.
When available, health assessors can refer to the toxicokinetic data analysis included in the MRL summary in the Toxicological Profile, which presents methods for extrapolating animal data to human exposure. You can review this summary to see whether any distinct differences between animals and humans have been documented for your contaminant. In absence of data to the contrary, assume that the toxicokinetics is the same in animals and humans under similar exposure conditions. These are some questions your evaluation might answer:
- Does the metabolism of the contaminant in animals produce more or less toxic intermediates than in humans?
- Do animals and humans produce the same metabolites in urine, feces, or exhaled air?
- Is the contaminant absorbed more or less in animals compared to humans?
Animals that have similar toxicokinetics to humans likely serve as good predictors of harmful effects in humans. Understanding the basic or specific biologic changes that ultimately lead to clinical disease in a test animal can help health assessors determine how well animal models might predict the similar adverse effect in humans. While this analysis is best done by toxicologists, reviewing documentation (e.g., Toxicological Profiles, IRIS) on biologic changes triggered by a contaminant can help health assessors evaluate the behavior of that contaminant at low doses. A toxicologist can further evaluate contaminant-specific factors by asking, for example, if the animal mode of action (MOA) is plausible in humans, taking into consideration kinetic and dynamic factors.
Several mathematical models can be useful in the health evaluation. Health assessors are not expected to conduct their own modeling, but having a general understanding of the model’s underlying principles can support your analysis. You can refer to the Toxicological Profiles and EPA IRIS for contaminant information that may be relevant to your evaluation. These are examples of the types of models:
- Physiologically based pharmacokinetic (PBPK) models estimate dose levels in body compartments and organs for a limited number of contaminants (e.g., lead, trichloroethylene [TCE]) where biologic uptake and mode of action have been well defined. For example, EPA’s IEUBK Model is a type of PBPK model that ATSDR health assessors should use when evaluating childhood exposure to lead in soil. This model predicts average and upper percentile blood lead levels for children (ages 0 to 7 years) exposed to lead in soil, while accounting for various other sources of lead and background blood lead levels in the population. PBPK models are also used to evaluate some chemical mixtures. Model inputs include the exposure dose and model parameters, such as tissue volumes, blood flow rates, partition coefficients, and metabolic rates. The output is the predicted internal dose (or target tissue dose).
- Pharmacodynamic (PD) models describe the quantitative relationship between the target tissue dose and cellular and molecular changes associated with adverse health effects. Increasingly, PD models account for damage, repair, and compensation, and predict dose-response over a range of doses, both within and between species.
PBPK or PD models can help reduce the uncertainty in the health evaluation. They also eliminate the need for cross-species extrapolation because they can account for differences in rates of biologic processes. The data used to support the model (e.g., metabolism and distribution data) can provide added perspective of how closely linked a particular dose might be to an adverse health effect.
Sensitive Populations and Life Stages
ATSDR recognizes that some populations (e.g., developing fetuses, infants, children, elderly persons, individuals with pre-existing health conditions like asthma) can be more sensitive to exposures in communities faced with environmental contamination.
Identifying or accounting for potentially sensitive and more highly vulnerable populations should also be a key component of your exposure pathway evaluation, and screening analysis. Also consider these populations as you estimate: site-specific exposure doses, adjusted air concentrations, hazard quotients, and cancer risks. The PHAST Exposure Calculator will assist you with this step, as it breaks out different exposure groups (e.g., birth to < 1 year, children aged 2 to < 6 years, pregnant women) so health assessors can separately evaluate health effects for more sensitive groups.
ATSDR places particular emphasis on populations that are sensitive to chemical exposure, such as children, pregnant and nursing women, and older adults. See this ATSDR fact sheet for more information.
At this point during the in-depth toxicological analysis, you need to determine whether special characteristics of the contaminant and of the site community might affect public health conclusions.
Remember that sensitive populations are considered when ATSDR and EPA develop health guidelines (MRLs, RfDs, and RfCs). An uncertainty factor (e.g., a factor of 10) is generally applied to help ensure sensitive populations are amply protected. Thus, when hazard quotients during your EPCs and Exposure Calculations Evaluation are below 1, it is highly unlikely that even the most sensitive populations would be adversely affected by exposure to that level of contaminant alone. ATSDR acknowledges that multiple chemicals and specific mixtures like polycyclic aromatic hydrocarbons (PAHs) and dioxins, can be either additive or synergistic in their toxicity. This topic of mixtures is discussed in the Multiple Chemical Exposures section.
Information on potentially sensitive populations can be found in the Toxicological Profiles (see Children and Other Populations that are Unusually Susceptible).
Factors that can make certain populations more sensitive to environmental exposures are described below:
- Age. Children differ from adults in their exposures and can differ in their susceptibility to certain hazardous contaminants. It is ATSDR policy that children’s health issues must be considered at all sites. (See box for special considerations when evaluating children’s health issues.)
Understanding when exposures occurred during critical development periods is important. This is particularly true for teratogens like TCE, which has been shown to cause fetal heart malformations in mice exposed in utero. Also crucial is knowing how a contaminant exerts its effect (i.e., its MOA).
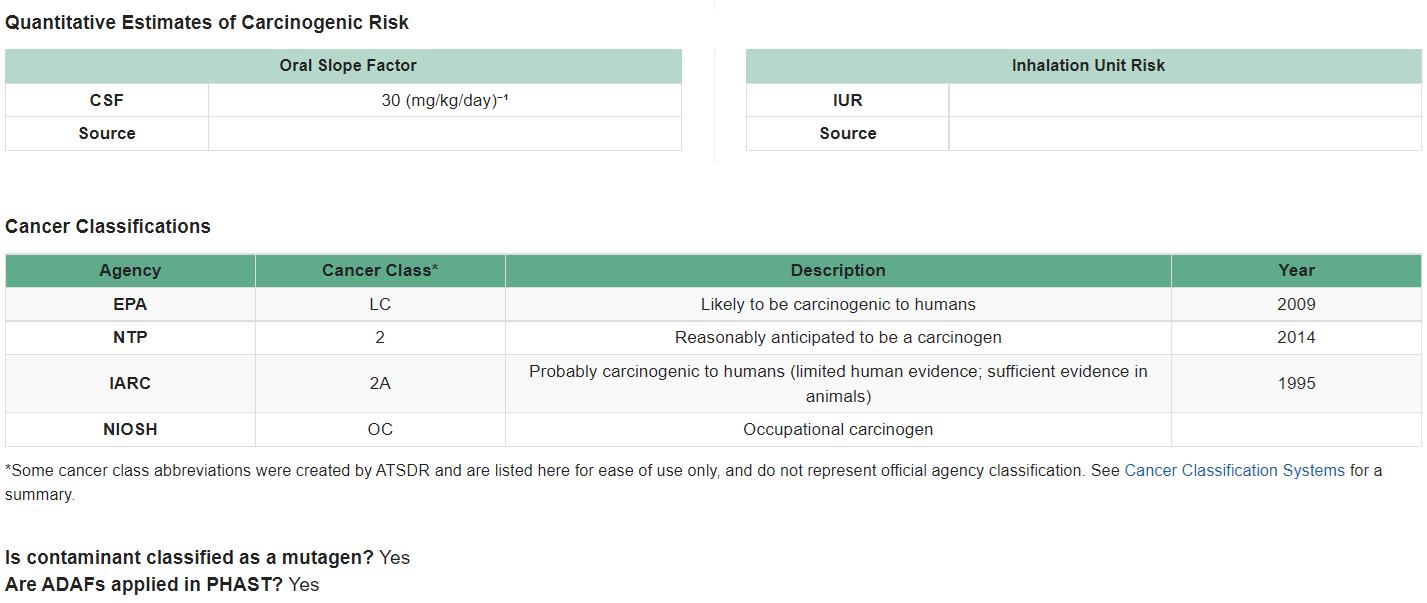
For instance, for chemicals that act through a mutagenic (DNA damaging) MOA, exposure during early-life stages can result in a higher cancer risk compared to the same exposure in children involving a carcinogen that is not mutagenic. EPA has identified contaminants that have a mutagenic MOA, and accounts for this in their CSFs and IURs used to calculate site-specific cancer risks. Health assessors can use the CVs and Health Guidelines Module within PHAST to identify mutagenic carcinogens. PHAST also provides other important cancer information, as shown in the example below for 1,2,3-trichloropropane.
When evaluating the possible public health significance of child exposures at your site, consider the following questions:
- What health effects have been observed in children? At what doses?
- Do epidemiologic or medical studies exist that suggest a special effect on children? Do exposure data exist?
- What health effects have been observed in adults exposed during childhood?
- What conclusions, if any, can be drawn from animal studies (are animal models relevant to children)?
- Do differences in pharmacokinetics/ pharmacodynamic parameters and metabolism make children more susceptible to a particular contaminant than adults?
- What is known about a contaminant’s characteristics (physical, chemical, toxicological) that would harm the developing fetus (e.g., can the contaminant or its active metabolites cross the placenta)?
- Can the contaminant or its active metabolites reach—and be excreted in—breast milk?
- Is the developmental process (e.g., neurological development) altered by the contaminant of interest?
- What is the critical window of exposure (e.g., during the prenatal or postnatal period)?
- Is information related to childhood cancer available (related to prenatal or postnatal exposure)?
To account for the increased susceptibility of children to mutagenic carcinogens, as illustrated in the EPCs and Exposure Calculations section, ATSDR applies age-dependent adjustment factors (ADAFs) to the cancer risk equation using the following adjustments:
-
- Children birth < 2 years 10
- Children 2 to < 16 years 3
- Children and adults 16 and older 1
For one mutagenic carcinogen, vinyl chloride, sufficient data are available to derive age-specific CSFs. These age-specific CSFs can be used to estimate age-specific cancer risks for vinyl chloride. EPA has proposed two CSFs: one for early life exposure involving children and one for adult-only exposure. These two CSFs account for differences in susceptibility between exposure that begins in childhood and exposure that begins in adulthood. Therefore, ADAFs should not be used for vinyl chloride because the risk from early life exposure is already a part of the CSF and IUR. The PHAST Exposure Calculator calculates cancer risk for people who have 33 years of exposure starting at birth. When calculating a site-specific cancer risk for vinyl chloride, contact the ADS group for assistance.
Elderly populations can also have significantly heightened susceptibility to some contaminants because of lower functional capacities of various organ systems, reduced capacity to metabolize foreign compounds, and diminished detoxification mechanisms. It is difficult to generalize, however, because of variations across individuals and different rates in biological system breakdown. Older individuals can also have much different exposures than younger adults and children.
As noted in the EPCs and Exposure Calculations section, for soil-pica health assessors should assume this practice exists if you have a scenario that could involve children exposed to contaminated soils. Young children and developmentally delayed children have a higher incidence of soil-pica compared to older children and adults, for whom soil-pica is rare. Soil-pica behavior is most likely to occur in young children as part of their normal exploratory behavior, with 4% to 20% of young children exhibiting soil-pica (ATSDR 2007 [PDF – 422 KB]). Among young children, those between the ages of 1 and 2 years have the greatest tendency for soil-pica behavior.
- Sex. Some contaminant-specific adverse health effects can be mediated by hormonal influences and other factors that are sex-linked. In general, sex-linked differences in toxic susceptibilities have not been extensively investigated. However, it is well documented that, because of various physiologic modifications in the body that occur during pregnancy, pregnant women and their developing fetus are often at significantly greater risk from exposure to certain chemicals (e.g., lead, TCE) than are other members of the general population. Pregnant women also could have more exposure than non-pregnant women because pregnant women have a higher breathing rate. Pregnant and nursing women also tend to have a higher water intake than non-pregnant women, leading to higher exposures.
- Genetic background or ethnicity. Research suggests that certain genetic factors can increase the risk of developing chemically-induced health effects. Factors that can affect the susceptibility of exposed groups include acetylation phenotype (i.e., fast versus slow acetylators), sickle cell trait, and glucose-6-phosphate (G6PD) deficiency (Rios et al. 1993). In addition, individual variability in the induction of metabolic enzymes could cause people to respond differently to the same environmental exposure. For health assessment purposes, the susceptibility of the most sensitive subgroups should be considered.
- Health and nutritional status. Understanding the location and characteristics of subgroups, such as the elderly and those of lower socioeconomic status, will help identify pre-existing health conditions (e.g., asthma, nutritional deficiencies) that might influence the impact of site exposures. Carefully note locations of schools, playgrounds, recreational areas, assisted living communities, retirement homes, or nursing homes on or near a site; they can be important indicators of potentially sensitive populations.
- Cultural practices. Various practices (e.g., subsistence fishing, geophagy, medicinal use of plants) can lead to increased exposures. Consider these factors as part of the previous scientific evaluation components.
Multiple Chemical Exposures
The approaches outlined in this e-manual focus largely on evaluating contaminant-specific and pathway-specific exposures (e.g., ingesting benzene in drinking water). In reality, exposures can involve multiple chemicals and occur through more than one exposure pathway. Approaches for evaluating the effect of multiple exposure pathways are discussed in sections on the screening analysis and EPCs and Exposure Calculations Evaluation. This section briefly highlights how to approach scenarios with chemical mixtures.
Because most hazardous waste sites contain multiple contaminants, the health effects of exposure to chemical mixtures can be a particular concern. Whenever there is a possibility of multiple chemicals in a completed or potential exposure pathway, examine the possible combined action of chemicals (e.g., additive, antagonistic, and synergistic).
A first step in understanding the potential significance of multiple chemical exposures is to read the Interactions with Other Chemicals section of the Toxicological Profile about any known interactions among the contaminants detected at your site. This section will sometimes point out possible interactions with alcohol or over-the-counter medications (e.g., aspirin). For example, 2-butanone is often found with other chemicals such as n-hexane and methyl-n-butyl ketone because they are used together in commercial and industrial applications. The neurological and hepatic effects of 2-butanone alone are minimal. Clinical reports, animal studies, and some in vitro tests have shown that 2-butanone potentiates or enhances these contaminant-related effects:
- the neurotoxicity of ethanol, n-hexane, methyl-n-butyl ketone, ethyl-n-butyl ketone, and toluene;
- the hepatotoxicity of carbon tetrachloride, chloroform, n-hexane, and dimethylformamide; and
- the renal toxicity of methanol and chloroform.
Thus, people who drink alcohol and are also exposed to 2-butanone from waste sites could be at greater risk for liver damage depending upon their dose of 2-butanone.
For many chemicals, however, this information is lacking, and the available literature focuses on the effects of chemical interactions at exposure doses much higher than those typically encountered at sites evaluated by ATSDR. Furthermore, even when information on chemical mixtures is available, no empirical data set could account for the infinite array of chemicals in varying proportions that can be found at sites.
ATSDR has a separate guidance manual (independent of this e-manual) – ATSDR’s Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors – that describes the agency’s three-tiered approach (i.e., Tier 1, 2, and 3) for evaluating exposures to multiple chemicals. The three tiers are:
- Tier 1. During the Tier 1 preliminary evaluation, you will compare exposure estimates based on sampling data with health guidelines and cancer risk values to identify exposure pathways and contaminants requiring further evaluation. In the Tier 1 preliminary evaluation, exposure estimates based on environmental media data are compared with health guidance values for single chemicals and chemical mixtures of concern to identify exposure pathways and chemicals requiring further Tier 2 or Tier 3 evaluation. Exposure pathways of concern are those with evidence that community members have, or are likely to, come in contact with a contaminant (e.g., drinking contaminated water, breathing in contaminated air, ingesting contaminated soil).
ATSDR’s PHA process and ATSDR’s Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors (the “Mixtures Framework”) provide different guidance, which complements each other. The PHA process is the agency’s main method for identifying potential contaminants of concern and possible exposure-related public health impacts. The Mixtures Framework helps ensure that the mixture of individual contaminants at a site are not an issue for public health.
The initial screening comparison of site-specific exposure estimates with health guidance values for single chemicals and defined mixtures are (1) the ratio of an exposure estimate to the health guidance value for noncancer health effects (the hazard quotient) and (2) the product of the exposure estimate multiplied by an EPA-derived cancer slope factor for carcinogenic chemicals (the cancer risk estimate). Agents with hazard quotients ≥ 0.1 or cancer risk estimates ≥ 10-6 are retained for further Tier 2 analysis. Single chemicals with hazard quotients < 0.1 or cancer risk estimates < 10-6 (e.g., 10-7 or 10-8) are not expected to pose health impacts individually or in combination with other chemicals and are typically not included in the Tier 2 analysis. An exception is cases where data and information collected for the site (e.g., community health concerns, industrial processes that use multiple chemicals) indicate that combined exposure to multiple chemicals could possibly result in adverse health outcomes.
Reviewing the Interactions with Other Chemicals section in the Toxicological Profile may provide insight for exceptions to HQ < 0.1. The HQ and CR results from the EPCs and Exposure Calculations Evaluation can be used to conduct the Tier 1 analysis.
- The Tier 2 analysis starts with a preliminary screening evaluation of non-cancer and cancer health effects from combined exposure to multiple contaminants. For non-cancer effects, you will use the Hazard Index (HI) approach. The preliminary hazard index is a sum of individual hazard quotients of all known contaminants for the site-specific exposure pathways of concern. This approach involves summing the hazard quotients for all contaminants with individual hazard quotients ≥ 0.1 and is based on the assumption of dose addition. The steps are to:
-
- Calculate an HI for the mixture of chemicals at a site. An HI is the sum of the individual hazard quotients calculated during the EPCs and Exposure Calculations Evaluation (i.e., HQ = exposure dose or adjusted air concentration divided by the non-cancer health guideline). The HI is calculated based on available MRLs, RfDs, or RfCs. Tier 2 is considered preliminary because the HI could be based on health guidelines with different endpoints. For example, the HQ for chemical one could be based on liver effects while the HQ for chemical two could be based on kidney effects.
The Framework for Assessing the Health Impacts of Multiple Chemicals and Other Stressors provides more information on calculating an HI and assessing multiple chemical exposures. More [PDF – 1,857 KB]
-
- Estimate separate HIs for each exposure pathway and exposure duration of concern. If the HI is less than 1.0 for individual pathways and durations, exposure to the specific mixture is likely not a health concern.
-
In scenarios where residents are exposed across multiple pathways (e.g., drinking water and air pathways), you also can sum HIs across multiple exposure pathways that affect the same population. The resulting multi-pathway HI gives an indication of the cumulative impact of exposure to a specific chemical mixture. This approach can be done for either chronic, intermediate, or acute durations.
In addition, the potential for chemical interactions that would lead to more-than-additive or toxic interactions (e.g., synergistic) should be evaluated. This topic is discussed in detail in ATSDR’s Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors.
For cancer effects, use the combined cancer risk estimate, which is a sum of cancer risk estimates of all carcinogenic contaminants of concern associated with a site-specific exposure pathway (i.e., contaminants with individual cancer risk estimates ≥ 1E-6).
- A final Tier 3 analysis is recommended when any of the following conditions are met:
-
- results of Tier 2 analyses indicate that site-specific exposure pathways have preliminary screening level HIs ≥ 1 or combined cancer risk estimates are ≥ 1E-6;
- concerns are high for additive or interactive joint actions (greater than or less than additive) from multiple site-specific contaminants of concern; or
- a health outcome data (HOD) review provides evidence of health effects possibly associated with exposure to site contaminants. Additional Tier 3 analysis steps will depend on the availability of data and resources (see the guidance for more information).
-
- For chemical mixtures with a Tier 2 HI greater than 1.0, health assessors may need to calculate target-organ-specific HIs. When the MRL or RfD/RfC are based on the same target organ, you can combine the previously calculated Tier 2 HI. If, however, the health guideline for each contaminant is based on different target organs, health assessors will need to calculate a target-organ-specific HQ for each contaminant. These target-organ HQs can now be added together to give a Tier 3 HI based on the same target organ. Under such circumstances, you should (1) calculate Tier 3 HIs for components with common target organ (see box on the right) or tissue effects or common adverse effects via a common MOA and (2) qualitatively evaluate information on possible interactions among mixture components as described in ATSDR’s Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors.
The TTD approach is a refinement of the Tier 2 HI approach. It is based on the fact that a chemical has the potential to cause multiple effects in humans and other organisms as a function of dose. Thus, as exposure levels increase, a chemical can affect multiple organs.
This approach can be used to assess mixtures whose components do not all have the same critical effect (i.e., the most sensitive effect providing the basis of the health guideline), but may produce toxic effects in secondary target organs dependent on exposure levels. The approach allows calculation of TTDs akin to effect-specific MRLs in Tier 3, as described in ATSDR’s Mixtures Framework. At hazardous waste sites, this approach can help provide a fuller characterization of health risks. TTD values could be similar to or higher than MRLs and can be used on a case-by-case basis.
-
- For chemical mixtures with a Tier 3 HI greater than 1.0, compare the estimated doses of the individual chemicals to their NOAELs or other observed-effect levels (LOAELs, BMDLs, HEDs) from the studies on which the health guidelines and target toxicity doses are based. If the site-specific doses or concentrations of one or more of the individual chemicals are more than an order of magnitude (i.e., one-tenth) of the respective point of departure (i.e., 0.1 x NOAEL, 0.1 x BMDL, or 0.1 x HED), conduct an in-depth toxicological evaluation to determine whether additive or interactive mechanisms could lead to harmful health effects. For example, the preliminary HI from Tier 2 might be based on liver effects for two chemicals and kidney effects for the third chemical. The health assessor will need to identify a target toxicity dose for liver effects for the third chemical that serves as a value akin to an MRL. The re-calculated (Tier 3) HI for the three chemicals is now based on liver effects for all three chemicals. A similar approach is used to evaluate kidney effects by using the target toxicity dose approach to identify a value akin to an MRL based on kidney effects for the two chemicals. Another Tier 3 HI is now calculated for the three chemicals that is based on kidney effects.
If the estimated doses of the individual chemicals are less than one-tenth of their respective NOAELs, BMDLs, or HEDs, significant additive or interactive effects are unlikely, and no further mixtures evaluation is necessary. In some instances, however, you might choose to further evaluate the potential for additive or interactive effects because of
- chemicals in the mixture having the same target organ (see box) or mechanism of action,
- exposures to potentially sensitive populations,
- uncertainty in the NOAEL/LOAEL/BMDL, or
- other reasons, such as strong synergistic effects.
In these instances, you can conduct an in-depth quantitative mixtures analysis as described above.
The health assessor needs to consider the potential for toxic effects from exposure to chemical mixtures at all sites. For more details, refer to agency guidance described in ATSDR’s Framework for Assessing Health Impacts of Multiple Chemicals and Other Stressors. Indicate that you evaluated exposures to chemical mixtures and considered the potential for chemical mixture interactions in your document.
Other valuable resources for information are ATSDR’s Interaction Profiles, which evaluate data on the toxicology of priority chemical mixtures. Interactions are deviations from the results expected because of additivity. Ultimately, the various types of interaction and noninteraction can be sorted into three categories: greater-than-additive (synergism, potentiation), additive (additivity, no apparent influence), and less-than-additive (antagonism, inhibition, masking). The Interaction Profiles incorporate ATSDR’s evaluations of the validity of particular studies and the inferences that can be made from them about interactions between chemicals in the mixture.
The intent of these profiles is to examine simple mixtures, which ATSDR defines as mixtures containing a small number of chemicals (no more than 10). The profiles evaluate the whole mixture data (if available), focusing on the identification of health effects of concern, adequacy of the data as the basis for a mixture health guideline (MRL), and adequacy and relevance of PBPK/PD for the mixture. The profiles also evaluate the evidence for joint toxic action—additivity and interactions—among the mixture components. The documents also develop target organ doses that can be used to evaluate the impact of the chemical mixture on different target organs.
ATSDR develops Interaction Profiles for chemical mixtures that are of special concern to ATSDR, such as:
The interaction profiles provide recommended approaches for examining possible non-cancer or cancer health hazards for populations potentially exposed to the mixture at a site. Health assessors can use these recommended approaches, along with incorporating site-specific exposure data and following ATSDR’s methodology for evaluating multiple chemical exposures, to develop site-specific health evaluations for mixtures. For more details on how ATSDR develops the interaction profiles, see ATSDR’s Guidance for the Preparation of an Interaction Profile [PDF – 841 KB].